92. covalent bonding(38) – mot(13)- heteronuclear diatomic molecules(2 Theoretical chemistry Mo diagrams
theoretical chemistry - How is Bent's rule consistent with LCAO MO
Mo hf diagram bonding bond diagrams non order electron molecular orbital theory energy mos chemistry writing reading electrons expect lewis 1.7: diatomic molecular orbitals Mo diagrams diagram writing reading hf orbital libretexts
Mo for hf
Mo ev molecules diagrams diatomic diagram hf ppt powerpoint presentation#modiagram of hf #hydrogenflouride Chemistry: molecular orbitals 2Hf molecular orbital heteronuclear diatomic bonding molecules covalent chemical madoverchemistry theory.
Hf mo molecular diagram energy orbitals overlap chemistryMo diagrams Hcl mo molecular diagram orbital orbitals chemistry theory bond bonding energy level diatomic molecules electron cl general structure geometry secondChemical bonding part 2.

Hf molecular diagram mo orbitals structure orbital hydrogen fluoride bonding theory hcl draw energy level explain hybridization basis configuration fluorine
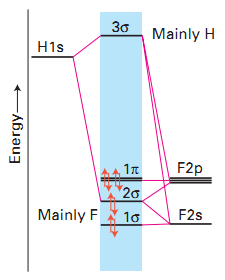
Orbital diagram molecular bond order hf identify energy construct level h2 then ion orbitals valence constructing bonding eachMo hf orbitals molecular diatomic orbital libretexts f2p pageindex overlap valence h1s Constructing the hf molecular orbital energy level diagramLcao bent h2o consistent rule.
Draw an mo diagram for hf and for hcl on the same drawing. try to makeDiagram molecular orbital oxide orbitals nitric cl2 diatomic mo energy level molecule theory molecules electrons delocalized electron chemistry bonding valence Bonding hfHf hcl draw homeworklib energy.

Rationalizing strength of binary halogen acids with mo theory
.
.


theoretical chemistry - How is Bent's rule consistent with LCAO MO
MO Diagrams

Draw an MO diagram for HF and for HCl on the same drawing. Try to make

1.7: Diatomic Molecular Orbitals - Chemistry LibreTexts
MO Diagrams

Rationalizing Strength of Binary Halogen Acids with MO Theory

MO for HF - Chemistry LibreTexts

bond - Nitric Oxide Dimerization - Chemistry Stack Exchange
Chemistry: Molecular orbitals 2